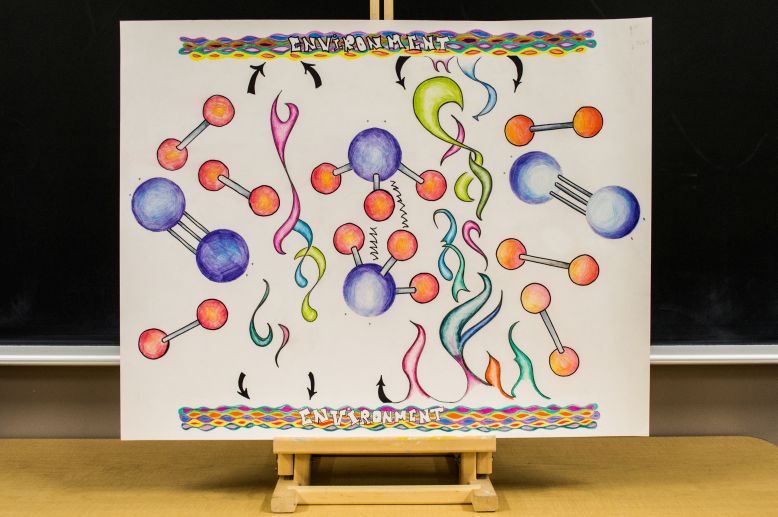
Atkins’s list of nine big ideas brings together all the notions of chemistry and subdivides them into nine categories. My goal in this project was to portray an upwards of four of these ideas by artistic measures.
The basis of my artwork is the formation and decomposition of ammonia. Right away, Atkins’s first big idea is evident: matter is made of atoms. Furthermore, the hydrogen atom and nitrogen atoms are clearly not the same size, which is a characteristic of their periodicity. Based on the placement of an element in the periodic table, its size, electronegativity, ionization energy, etc. are assigned. Hydrogen is at the top left corner of the periodic table, having the smallest radius, whereas nitrogen in across the table, with a considerably larger radius. I demonstrate periodicity through the size of the atoms, which is dictated by their position in the periodic table of elements.
Another of Akins’s big ideas is chemical bonding. This is a very important part of chemical reactions and is demonstrated in my artwork through the formation and decomposition of ammonia. To form ammonia, the bonds in the hydrogen and nitrogen molecules must be broken and new bonds must be formed. Chemical bonds form when electrons pair. Each bar in the image represents a pair of electrons, and the dots above the atoms are lone pairs of electrons.
The next big idea I chose to demonstrate is the molecular shape, which, as Atkins states, is a crucial feature in chemistry. This is evident in the shape of the ammonia molecules in the center of the image. The shape of the molecule is dictated by the number of lone pairs and paired pairs of electrons, as well as the hybridization of the central atom, nitrogen. Nitrogen has the hybridization sp3 because there are three hydrogen atoms attached to it and it has two lone pairs. This gives the molecules its tetrahedral orbital shape (the molecular shape is trigonal pyramidal). The lone pair of electrons is situated on the arbitrary ‘top’ of the molecule, and the hydrogen atoms are separated by angles slightly smaller than 109.5°.
Thereafter, the conservation of energy is incorporated into the drawing. I illustrate both the formation and decomposition of ammonia expressly for this purpose. I drew colorful bands above and underneath the reaction to represent the environment. In this picture, color is energy. I represented it with swirls and shapeless forms because my interpretation of energy is of an abstract colorful ever changing being that is alive, hence the bright colors and amorphous swirls. The goal of having the environment bands on the edges of the image is to demonstrate that energy is released in the first reaction (formation of ammonia is exothermic) and enters the environment, but the opposite reaction absorbs energy from that same environment, indicating that energy is never lost or created, it is always released or absorbed from the environment.
For my sixth idea, I chose to showcase Atkins’ big idea of residual forces between molecules. To do this, I illustrated hydrogen bonds that occur between the nitrogen and hydrogen atoms in the two separate molecules of ammonia. This force is depicted as being a zigzagged line because I envision intermolecular forces as chaotic pushes and pulls of atoms, as opposed to easy attraction where the molecules gravitate towards each other; my intention was to demonstrate a sort of aggressive action.
The final idea exemplified in my art project is that there are barriers to reactions. As I had earlier stated, the formation of ammonia is exothermic whereas the decomposition is endothermic. The exothermic reaction necessitates less energy than the endothermic, meaning that its activation energy is lower. What this entails is that less energy must be inputted to perform the formation of ammonia whereas a large amount must be entered for the decomposition, proving that there are barriers to reaction. I demonstrate this through the amount of colors between each reaction. Because the formation has lower activation energy, there are less colors and shapes between the reactants and products, in contrast with the decomposition.
In conclusion, with the aid of seven of the nine big ideas in chemistry, I have demonstrated the formation and decomposition of ammonia while incorporating my artistic interpretations.