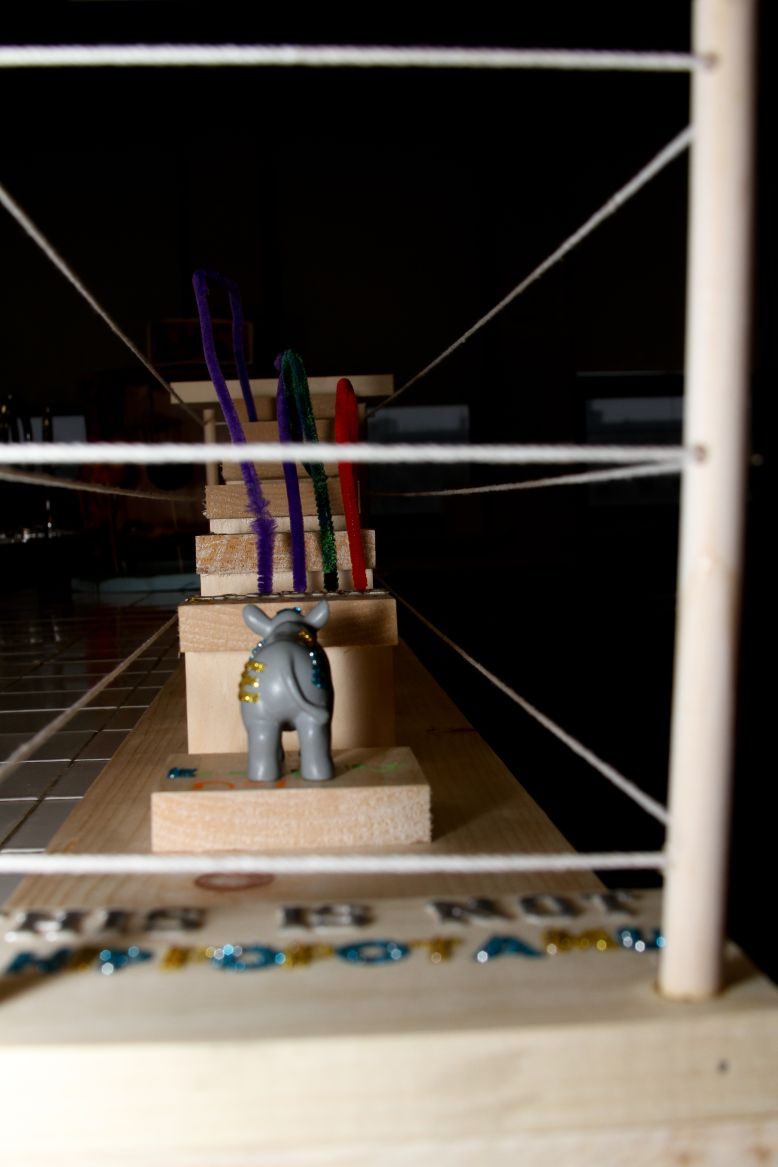
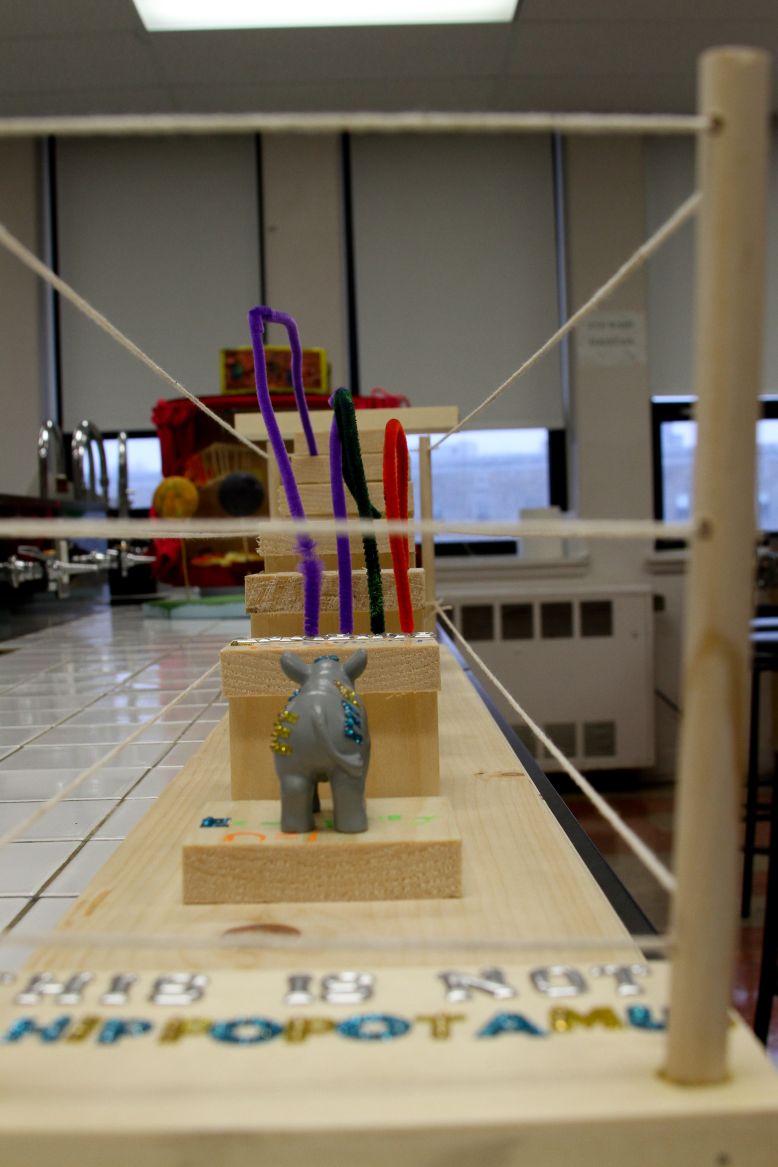
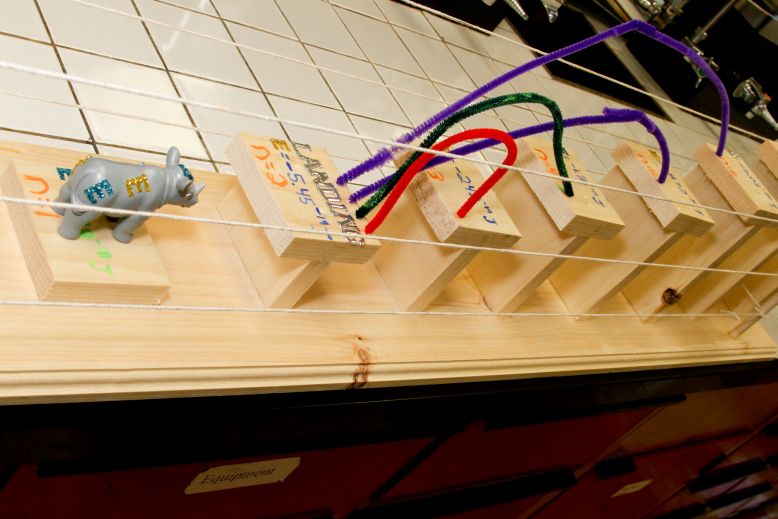
Once Schrödinger’s equation was settled, we were able to come up with a graphical correspondence of the orbitals that represent the wave functions. In the atomic spectroscopy, each principal quantum number has a unique amount of energy that is required for an emission or absorption. Each wavelength represents the emission or absorption of an electron between quantum-mechanical orbitals and for that line to be visible, the wavelength must be between 400-700 nm. In my art project, I represented the emission spectrum of a hydrogen atom for the visible wavelengths which are from n=3-n=2 (656 nm), n=4-n=2 (486 nm), n=5-n=2 (434 nm), n=6-n=2 (410 nm), and as for n=7-n=2 (397 nm), I represented the energy level but didn’t represent the emission line since it’s hardly visible. We can almost consider it as ultraviolet. I also represented n=infinity. I represented the visible lines with the colors that I could find that were the closest to the real colors and indicated landing on the second level to show that it is emission and not absorption. I decided to use a hippopotamus to represent my electron since it makes no sense at all for a hippopotamus to jump from one level to the other just like the fact that quantum is totally against common sense. I also indicated this is not a hippopotamus to show that it is only the representation of a hippopotamus and not the actual animal. The purpose of the poles and ropes that I inserted around my project is to show that once the electron has had the required energy to be ionized (n=infinity), which is the opposite of the ground state (n=1), it is free. I also put gaps between each level (or stage) because if the electron doesn’t have the exact energy required for that transition, it will not be able to accomplish the jump. So basically, my art project is the representation of the emission spectrum of a hydrogen atom for the visible transitions.